

FEEL CONFIDENT IN YOUR ACCESS TO PARSABIV® FOR
CLINICALLY APPROPRIATE PATIENTS WHO NEED IT.
Understanding ESRD reimbursement to enhance planning for patient care
Transitional Drug Add-on Payment Adjustment (TDAPA) was established by CMS to make and keep qualifying new drugs and biologicals accessible to CMS beneficiaries3
According to CMS, “The intent of TDAPA is to facilitate beneficiary access to certain qualifying new injectables or IV products, allowing payments for these drugs and biologicals while the necessary utilization data is collected.”4
TDAPA process
When a therapy is added to the ESRD PPS bundle, its valuation is based on utilization under TDAPA5
Average Parsabiv® utilization of
~6.3%of dialysis sessions in Q3 2018-Q4 20192
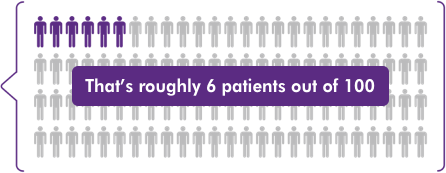
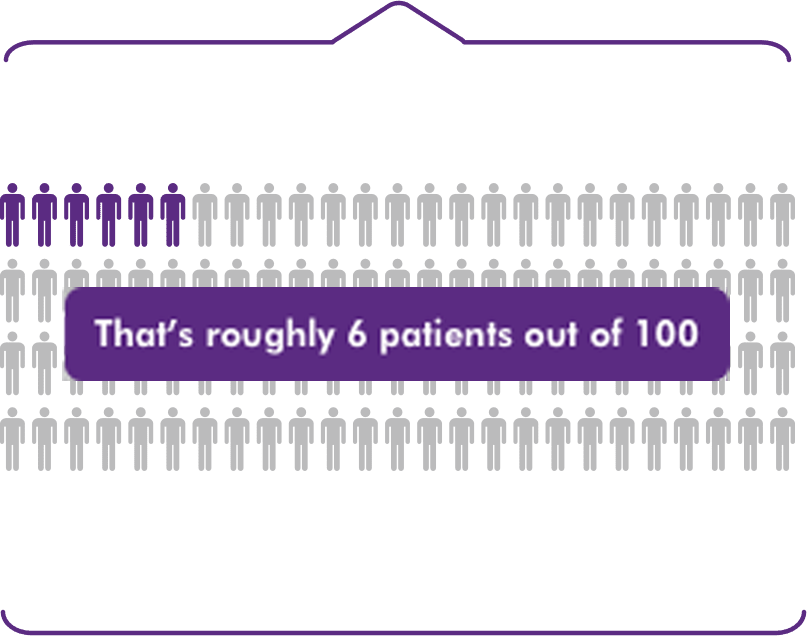
That’s roughly 6 patients out of 100
Amount added to the bundle for calcimimetics, including Parsabiv®, in 20211
The addition means providers receive the same $10.09 calcimimetics reimbursement, whether patients are taking Parsabiv® or not1
The additional reimbursement for Parsabiv® (etelcalcetide) based on the Q3 2018-Q4 2019 national average of ~6.3% of dialysis treatments6 supports continued access to Parsabiv®.
supports patient access to Parsabiv®.
Reflects a $9.93 calcimimetic valuation plus a market basket increase of 1.6%.
CMS = Center for Medicare and Medicaid Services; ESRD = End-Stage Renal Disease; PPS = Prospective Payment System.
Reimbursement for Parsabiv®, and other dialysis costs, is adjusted annually by a market basket increase to the ESRD bundle rate.1
Periodic rebasing of the ESRD market basket occurs to reflect a more up-to-date cost structure7
.

Frequently asked questions
How are different organizations being impacted now that Parsabiv® reimbursement has been added to the bundle?
Now that Parsabiv® is reimbursed as part of the ESRD bundle, some organizations are limiting access. Dialysis organizations can think about reimbursement across all dialysis patients since the additional amount for calcimimetics is there for every single dialysis session, not just for dialysis sessions for patients prescribed Parsabiv®. Based on utilization at ~6.3% of dialysis sessions during the evaluation period, the $10.09 added to the ESRD bundle is designed to cover Parsabiv® for clinically appropriate patients. This amount is expected to grow annually with the bundle base rate increase.
How does being in the bundle impact patient out-of-pocket costs?
The portion of the bundle not covered by Medicare will increase by the same percentage that the bundle increases to account for the cost of Parsabiv® and other dialysis costs. Reimbursement for Parsabiv®–and other dialysis costs–is adjusted annually by a market basket increase to the ESRD bundle. This will likely impact those patients who don’t have a secondary payer source. This Medicare policy is not specific to calcimimetics.
What can I do to get Parsabiv® for patients who really need it?
Talk with your medical director about the patients for whom Parsabiv® is the best fit, and make sure they and your care team understand Parsabiv® reimbursement. You can also reach out to your Parsabiv® representative on how to help start a conversation with your medical director for them to understand the reimbursement dynamics of Parsabiv®.
Contact your Parsabiv® account manager for more information about reimbursement and how we can support you and your care team.
Other payers
Medicare Advantage (MA)
MA plans receive capitated payments to provide all Medicare‐covered services to pay enrollees, and they are responsible for covering calcimimetics for clinically appropriate patients. Coverage and reimbursement will vary based on an individual organization’s payer contracts. Reach out to your contracted payer or parent organization to determine how Parsabiv® will be covered. Getting coverage for Parsabiv® may require amending your dialysis organization’s payer contracts. Work with your contracting group to make the case for coverage for clinically appropriate patients when contracts are being negotiated.
Medicaid
Medicaid coverage varies by state. Check with your State Medicaid Office to understand coverage and requirements specific to your state.
Commercial
Commercial coverage and reimbursement will vary. Verify payment details once you have identified clinically appropriate patients. Reach out to your contracted payer or parent organization to determine how Parsabiv® will be covered under your specific contract terms; contract amendments may be necessary. Work with your contracting group to make the case for coverage for clinically appropriate patients when contracts are being negotiated.
SUPPORT AND SERVICES
We’re here and ready to help.

Personalized support that you and your patients can count on across Amgen therapies.
Amgen SupportPlus Representatives
Our Amgen SupportPlus Representatives can assist with issues around patient coverage, prior authorizations, and more.
Amgen Financial Support
We know every patient has unique needs. And we're here to provide financial support information and resources, regardless of their current financial situation or type of insurance they have.
For financial support information and other resources for your patient, contact Amgen SupportPlus at (866) 264-2778 Monday through Friday, 9:00 am to 8:00 pm ET.
EFFICACY
See how Parsabiv® performed
in clinical trials and its use in real-world outcomes
DOSING & MONITORING
Switching from oralcinacalcet to Parsabiv® (etelcalcetide)
REAL-WORLD EVIDENCE
See an analysis from
hundreds of real-world treatment experiences



